Electrical Conductivity
Electrical Conductivity (EC) is a measure of the ability of water to pass an electrical current. Conductivity in water is affected by the presence of inorganic dissolved solids such as chloride, nitrate, phosphate and sodium.
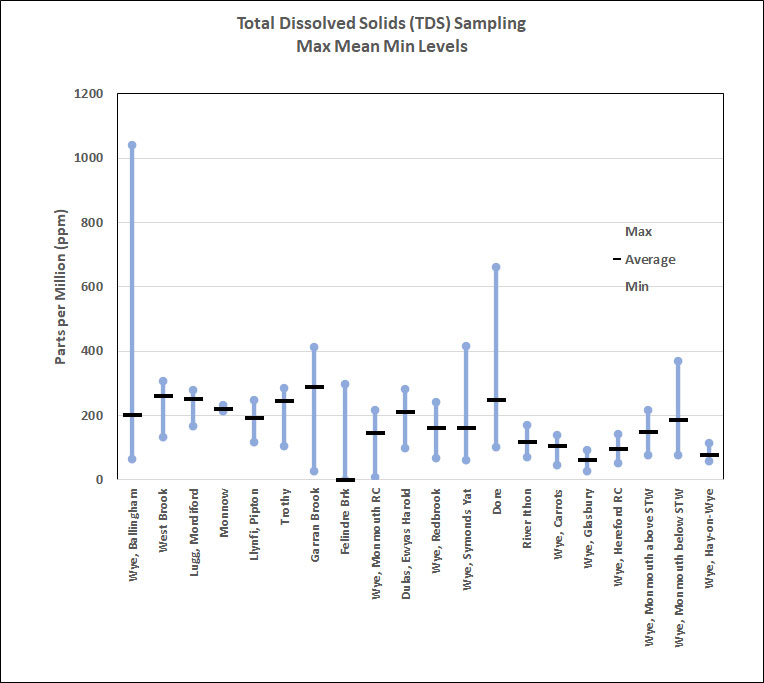
Total dissolved solids (TDS) is a measure of the combined inorganic and organic substances dissolved in water. It is directly related to the electrical conductivity of water and as such can be used as an indication of change.Organic compounds like oil and alcohol do not conduct electrical current very well and therefore have a low conductivity when in water. Conductivity in streams and rivers is affected primarily by the geology of the area through which the water flows. Streams that run through areas with granite bedrock tend to have lower conductivity because granite is composed of more inert materials. Streams that run through areas with clay soils tend to have higher conductivity because of the presence of conductive minerals that are washed into the water from the clay. Discharges of pollution into streams can change the conductivity depending on their make- up. Sewage from a missed plumbing connection would raise the conductivity
because of the presence of chloride, phosphate, and nitrate; an oil spill would lower the conductivity. TDS is measured in ppm, rivers generally ranging from 35 to 1050ppm. Rivers supporting good aquatic life have a range between 105 and 350ppm. TDS outside this range could indicate that the water is not suitable for certain species.
TDS is converted from EC with a typical conversion factor of 700:1. This ratio means that a single unit of EC equals approximately 700 ppm